Designing a decentralized clinical trials registry
Discovery and design of a DTRA industry registry to monitor adoption and support evidence generation of the impact of Decentralised Clinical Trial methods on study conduct and performance.
The Challenge of Demonstrating Consistently the Evidence of Impact of DCT Methods across the Industry
The DTRA engaged with Windmill Digital, to support them in the Discovery, Design Thinking led Sprints and Prototype Design of a central DCT intelligence solution for its Biopharmaceutical members. The solution would:
- Allow Pharma sponsors to track their studies using decentralized methods
- Enable sharing of specific data tracking which studies use decentralized methods
The project was a high priority request for the DTRA and its members, as there is an urgent need to generate Evidence of Impact of decentralized research methods on study conduct and performance.
- Most Bio Pharma sponsors have no existing tools that allow the consistent & standardised tracking of which studies use DCT methods
- Agreed and standardised cross industry benchmarking and other data on DCTs, is critical for demonstrating impact and sustaining continued adoption with both Bio Pharma sponsors and regulatory agencies.
Why DTRA Chose Windmill Digital
The DTRA enlisted the Head of the Windmill Digital Healthcare BU, as a Pharmaceutical Executive consultant with experience in global study management and DCT innovation, pharmaceutical data & business intelligence and digital solution experience.
The Windmill Digital portfolio includes the vast experience of designing and developing digital experience products utilising and visualising complex data and analytics, including pharmaceutical intelligence and clinical trial benchmarks. Previous projects delivered demonstrated the capability of digitising workflows and bringing to life complex data for decision making, especially in planning, forecasting, benchmarking, analysing, and reporting.
How Windmill Digital Responded
Windmill Digital engaged with the DTRA and its BioPharma Sponsor members, in an intense discovery process to assess the industry & competitive landscape. The Windmill team determined BioPharma sponsor company requirements via in-depth interviews and group workshops, which included a very productive MOSCOW prioritization approach (MoSCoW stands for “must-have,” “should-have,” “could-have,” and “won’t-have (this time) to support product prioritisation and gain alignment for MVP and potential future roadmap.
The Windmill team also helped pressure test the value proposition of the proposed solution to ensure it was fit for purpose for current and future evolution of industry need.
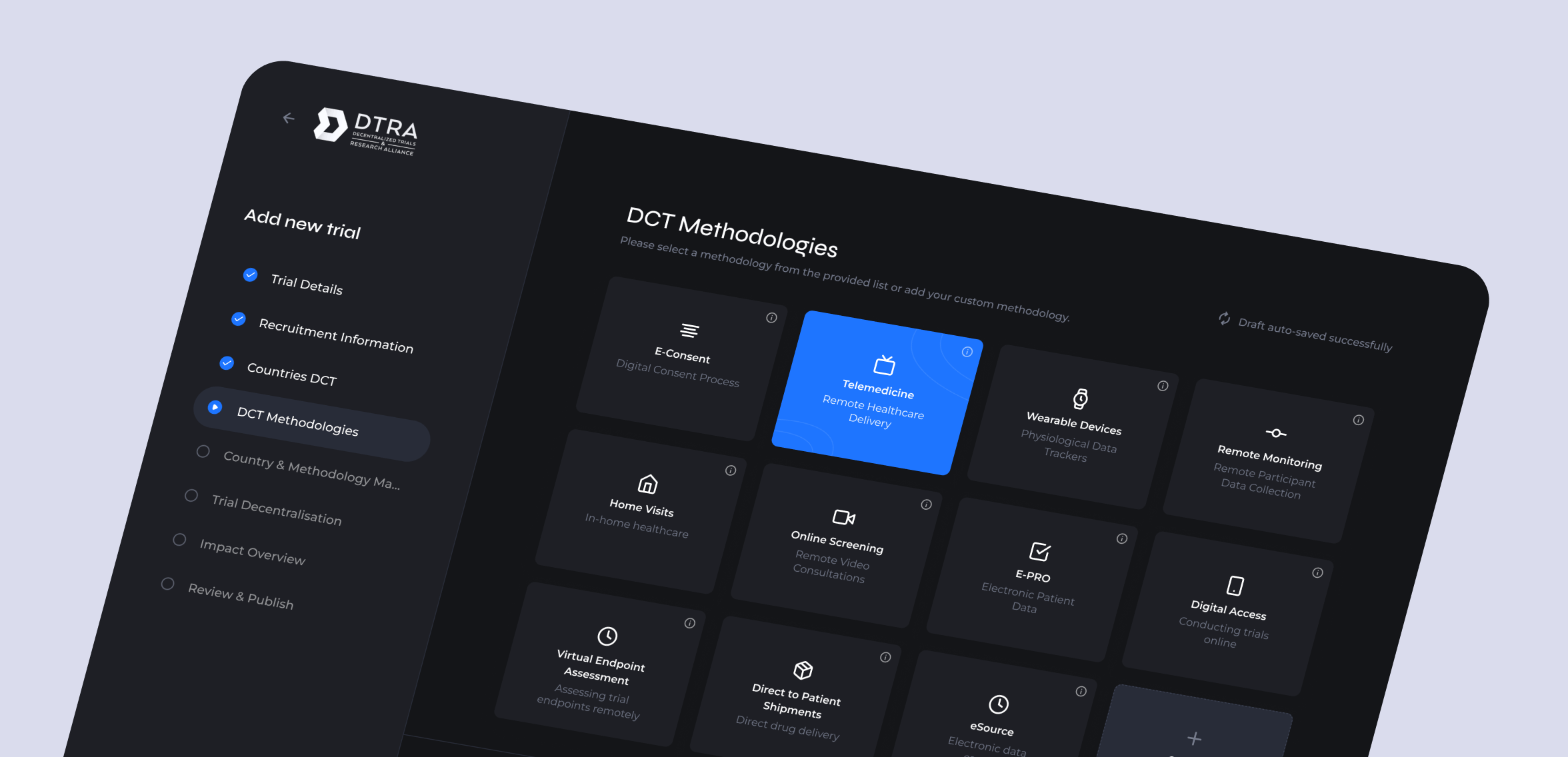
An interactive prototype was designed that would allow BioPharma sponsors to centrally and securely track their studies using decentralized methods with an opportunity for a white-labelled front-end for each pharma sponsor organization.
The created designs also provided new-industry first IMPACT measurements, tracking adoption of pre-specific decentralised methods against study types, and per country deployments for assessing geographical as well as modality trends.
The prototype created would allow for centralisation of specific data tracking at the aggregated data level, as well as learning and knowledge sharing of the types of DCTs methods suitable for which studies and patient populations to aid in confidence build of DCT adoption.
The Results
“The Windmill team leveraged their consultative expertise together with Design Thinking methodology to help clarify the varied industry and customer needs across BioPharma sponsors. A live protoype was rapidly built to support end user experience and a very productive MoSCoW prioritization approach helped achieve alignment quickly across the stakeholder group.”
Jane Myles, Program Director, DTRA
